Carbon dioxide is a critical factor that determines, for many, success or failure with planted aquariums. Making sure you are providing enough to your plants given your unique aquarium situation can be challenging. In this episode, we dive into the science side of planted aquariums and discuss the importance of CO2 and how to go about making sure you are providing enough.
In Aquascaping News, I mentioned Interzoo, the 2014 IAPLC, the Aquatic Gardeners Association 2014 Aquascaping Contest is now taking entries (deadline is September 15th and remember they have a Dutch style category), and the latest issue of The Aquatic Gardener (TAG).
This issue of TAG has a writeup on the 2014 Art of the Planted Aquarium contest and the winners of the qualifiers. There’s a reference to a great video of the legendary Ole Pederson explaining why one of the contests in the Nano category ended in third place. There’s also a wonderful article on how aquascaping is growing in Spain very similar to the one I did on what’s happening in Brazil. Good stuff!
I have to mention the amazing article Takashi Amano has entitled a Distant View Created by Stacking Yamaya Stones. Go take a look at it. See what it looked like recently planted and when it filled in. I just don’t have that vision. It’s a gift.
The issue wraps it up with an article on Crypocoryne cordata var. siamensis by two legends, Niels Jacobsen and Jan Bastmeijer and a New Aquascape of Polish aquascaper Piotr Dymowski by our Brazilian friend, Luca Galarraga (who also was at Interzoo having fun).
Lastly, Aquavas released a new video. It’s beautiful. Go have a look.
How do you know you are providing enough CO2?
Unlike oxygen (O2) molecules that remain O2, whether in its gas form or in solution, carbon dioxide (CO2) will react with water (H2O) to form other compounds such as carbonic acid (H2CO3). You don’t need to be a chemistry geek and figure out the formula. It’s not that important. Simply understand that CO2 may transform itself into other forms in water if certain conditions exist.
Recap of pH in aquarium water
To understand what happens when you add CO2 to the aquarium, you need to first have to know the relationship between pH and water hardness. Now, if you remember from grade school, the power of hydrogen (pH) is the negative log of the hydrogen ions or (pH = -log [H+]). It can range from 0 to 14 with 7 being neutral. Anything below 7 (having more H+ ions) is considered acidic and anything above 7 (having less H+ ions) is considered basic.
Now, several things can impact the pH of our aquarium water. These include biological nitrification (decreasing pH) and photosynthesis (increasing pH). It’s not important to focus on maintaining a strict pH in our aquariums. What matters is that we stay within a targeted range and have a natural, non-detrimental pH swing.
So now you know that pH is impacted by the number of H+ ions in the water and that several processes can increase or decrease that number. You now need to understand water hardness and its relationship with pH in your aquarium.
Water hardness in aquarium water
General hardness (GH) refers to the water’s relative concentration of calcium (Ca) and magnesium (Mg) ions. Carbonate hardness (KH) refers to the amount of free bicarbonates (H2CO3) and carbonates (Co3) anions and is also commonly known as alkalinity but that’s a bit of a misnomer. Alkalinity is a measure of the ability of water to resist a decrease in pH upon the addition of an acid. Alkalinity can be provided by any number of compounds (carbonate, bicarbonate, borate, phosphate, hydroxide). A better way to understand KH is what Dr. Morin of Seachem calls “temporary hardness” because changes in CO2 concentration and acid levels can rapidly affect this value.
Soft water is a term used to describe water that has a low GH and KH. Hard water refers to water that has a high GH and KH. Soft water will typically have a lower pH, while hard water will have a higher pH. However, tap water doesn’t always follow this generalization. Therefore, testing the water hardness of your tap water and determining GH and KH is important. Make sure to get yourself a test kit with an expiration date on it from a reputable company. Yes, they may cost a little more, but at least you know what you’re getting. I’ve never been so mad as the time I made a bunch of frantic changes to my aquarium only to find out later that my test kit was bad.
Plants do have a preference for hard or soft water depending on where they come from. Although I’ve found that most plants tend to adapt themselves to a neutral range, there are some that definitely won’t do well unless you provide them with the conditions they’re adapted to.
Here’s a great video that explains pH and water hardness very well: Aquarium pH and Water Hardness
So how does this relate to CO2?
Well, an acidic (low pH, GH and KH) environment will have a higher concentration of dissolved CO2, while an alkaline environment (high pH, GH and KH) will have a lower concentration of dissolved Co2 in the water. Therefore, plants that prefer soft water (e.g., many of our aquarium plants) and supplemental CO2 is a must to keep them growing well.
Here’s another great video that explains this: Aquarium Plants and Carbon Utilization
OK, so assuming we are supplementing CO2 in order to increase the acidity of our water and the availability of it within the water column, let’s understand what happens to it in solution. When CO2 is added to water, it becomes surrounded by water molecules and is forced into an equilibrium with different forms of carbon. We refer to the result as dissolved inorganic carbon (DIC).
- As a first step, CO2 mixes with water to form carbonic acid.
- It is short lived and quickly becomes bicarbonate. This process releases hydrogen ions and this is why you see a decrease in pH when adding Co2.
- The last step that we won’t typically see in our aquariums is carbonate. This is because the pH needs to be quite high in order for it to form.
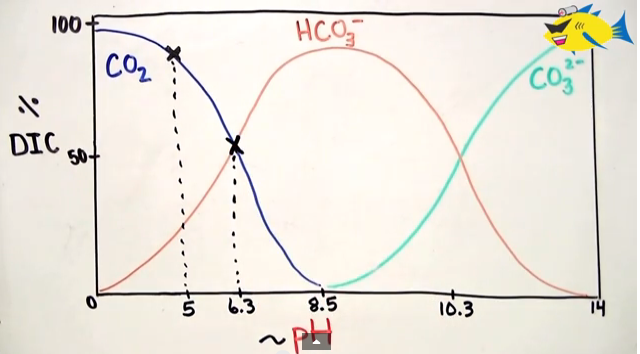
So the formula that I remember is CO2 <==> H2Co3 <==>HCO3 <==> CO3. This formula is influenced by the pH of the water. So at lower pH we see DIC in the form of CO2, equilibrium with H2CO3 is at about 6.3, and CO2 being completely absent at about 8.5. See the image below taken from DIY Aquapros’ video.
Here’s the wonderful DIY Aquapros video on this: CO2 Water Chemsitry in Planted Aquarium
So all things being equal, we now know that adding CO2 will decrease pH and that to make sure it exists in an easily available form for our mostly soft water plants, we need to keep the pH within a certain range.
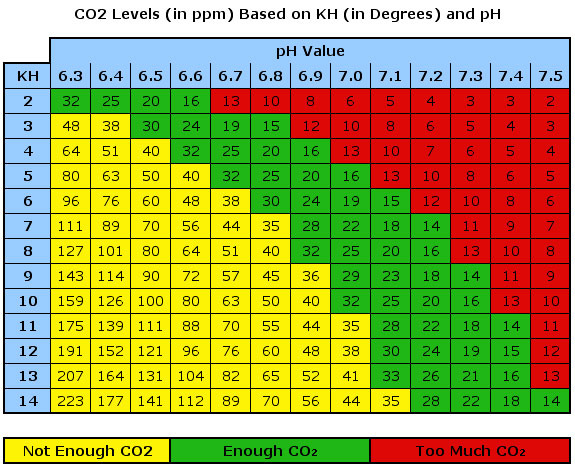
Here’s the famous table we all refer to indicating the relationship between KH, pH and CO2 concentration:
This table shows that for a given KH and pH level, a specific amount of CO2 should be available. We know from experience that a 30 ppm CO2 concentration is what we want for healthy plant growth. Therefore, we need a KH of 4 and a pH of 6.6.
But how to measure how much CO2 we are adding?
The problem we face is that there are many things that can impact the pH and KH of our aquarium water. There really is no way for us to practically keep these two static so that we can determine how much CO2 is present in the water column on a consistent basis.
Wouldn’t it be great if we could find some way to hold pH and KH steady so that we could isolate CO2? This way we would have a very good way to determine how much CO2 is available to our plants. In the old days, when I started in the hobby, it was very common to use a pH controller to control CO2 release into the aquarium. You would set a pH of say 6.6 using the table and you adjusted your aquarium water to a KH of 4. The release of CO2 via a solenoid valve was based on the pH of the water. If it went over 6.6, CO2 was added to bring it down to 6.6. The problem was that other factors besides CO2 in the aquarium worked to decrease pH and/or to change your KH so the accuracy you were hoping for was gone.
There came a solution, however, in the form of a very simple pH test using our old friend, bromothymol blue. You see, bromothymol blue or BB, as I like to call it, changes color based on the pH of the solution. It’s blue in an alkaline solution, green at around 6.6 and yellow in an acidic solution. If we had a way to use a stable 4 KH solution and add BB to it, we could see the effect of CO2 on the color if we were able to make sure ONLY CO2 entered the solution. We need some sort of barrier that will only let CO2 into the solution and nothing else that will impact pH or KH.
The answer, of course, was air. CO2 degasses easily into a gas seek equilibrium so if we had an air barrier between this BB solution and the aquarium water, CO2 would flow back and forth seeking equilibrium. This would allow us to determine the amount of CO2 in aquarium water by watching the color of the solution. But how to create this air barrier?
The answer is an upside down bell configuration. We’ve done it many times when you take a cup and push it down into a body of water. Air gets trapped in the cup when underwater. Brilliant! The drop checker was born to serve this purpose. Now we have a way to maintain a pH and KH steady with CO2 being the only variable, thereby allowing us to measure the CO2 in our aquarium water.
The art of measuring CO2 in a planted aquarium
Even though we’ve found a way to have a good indication of CO2 level using a drop checker, it isn’t extremely accurate because using color is more about getting to a range than a specific figure. However, that doesn’t really matter to us. It’s about making sure you have enough CO2 in the water for good plant growth while having less than the amount that causes your fish and shrimp from suffering.
This amount is different for every aquarium. There’s an art to it. You must find it by dialing it in. Slowly. Here’s how:
- One hour before lights come on, start a 1 bubble per second rate.
- Three hours after lights come on, see what color the drop checker is showing? If it’s blue, go to 2 bubbles per second. If it’s yellow, bring it down to a bubble every two seconds.
- Five hours after lights come on, check the drop checker again and see what color is showing. You a looking for a nice green color. Adjust the bubble rate up or down depending on the color. Make small adjustments. Keep an eye on the fish and shrimp and make sure they are not suffering.
- After a while, you will get to a rate that achieves green color an hour or two into the light phase of your aquarium and stays that way through the rest of the light phase.
- I turn off my CO2 two hours before the end of the light phase of my aquarium. By then, the plants are winding down and taking up less CO2.
- Once you have achieved this consistent green, try to take it up a notch. I try to find a green-yellow color where plants respond well but that doesn’t stress the fish and shrimp. Do I care to know the exact amount using the table? No. I know that green-yellow means I have enough CO2 given that I isolated CO2 to be the only variable as we discussed above.
Now, some tips.
- Place your drop checker towards the bottom of your aquarium and in a place of slow flow. This tries to ensure that those places that receive little CO2 have enough.
- Use a high flow powerhead that moves a lot of water but doesn’t create a jet such as the EcoTech Marine Vortech pumps
- Use a solenoid valve and simple timer to turn CO2 on and off rather than leaving it on all night.
- Clean your CO2 diffuser and drop checker with every water change.
- Dial in your CO2 bubble rate on a weekend when you can check it often.
- Spend the money and buy a reliable CO2 regulator, needle valve and solenoid.
- Be patient. You will get to know your aquarium and how it reacts so dialing it in and adjusting will be easier over time.
- You will need to adjust the CO2 bubble rate as your aquarium matures. There is no set and forget it. That’s the point.
In the From the Forums segment, this week is all the way from the UK Aquatic Plant Society – Twinstar what is it?. Twinstar is a new product from a South Korean company that claims to inhibit algea growth and disease in planted aquariums. The site is awesome and the product is used by the likes of David Chow, Oliver Knott and Viktor Lantos, some of the world’s best aquascapers. That alone makes me take notice. The 28 page (at the time of this episode) thread in UKAPS tries to understand what the product is because the site really doesn’t explain too much other than pretty amazing benefits.
The thread unearthed the patent for the product and it turns out it uses electrolysis to sterilize the aquarium water and convert certain nutrients into a more easily available form. This explains the incredible bubbling that it creates that’s due to the separation of O2 and H2 in the water. This must certainly oxygenate the water providing some obvious benefits. The sterilization must happen by killing algae spores but I assume this also kills floating bacteria that could include beneficial bacteria. There are some patents for similar products designed to replace a biological filter by bringing ammonia down to nitrogen chemically using electrolysis. So, if this does kill beneficial bacteria but takes waste out of the aquarium via a chemical path, do we care? Well, our plants use ammonia as a nutrient so, maybe, yes?
Also, I understand electrolysis in water will precipitate out calcium carbonate and magnesium. This may explain why people in the thread are complaining about build up on the product itself. I believe switching the polarity between the two plates every so often is supposed to help with this but it doesn’t seem to be working given the feedback in the thread.
In the thread, Viktor advises that this product works but that it’s not for beginners. You must have your aquarium maintenance down pact first because this product can’t fix lack of knowledge or lazy. However, if you know what you’re doing, this product can give you a level of buffer or cushion so if you are unable to do a scheduled water change or miss some dosing, your aquarium won’t crash. I assume it must also create crystal clear water.
I remain concerned about the impact on nutrient levels and the precipitation on the product itself. Putting aside the thought of running a current, albeit low, through the aquarium. If you’re using EI and doing your weekly water changes, I would suspect the impact on nutrients is negligeble. However, if you use another system such as ADA or PPS that rides with lower nutrient levels, I’d be a bit concerned and would wait for further feedback.
Nevertheless, it’s a very interesting product that I would love to test in my aquarium when it reaches the US. If you use it, I’d love to hear your thoughts on it. Send me an email at art@scapefu.com.
Finally, in Focus on You segment, we read some feedback. Special thanks to those of you that sent messages encouraging us to keep recording and even offering to help with the audio production. As you probably, heard, the last episode had some audio issues. Fortunately, we were able to sort them out by buying new and better equipment. Hopefully, you’re finding this one is sounding much better. Anyway, thanks to all who sent very helpful emails.
First up is an email from Giuseppe. He writes:
Hey Art,
I’ve been following your podcast for awhile now. I really enjoy it. Please keep it up. I’ve been in the hobby regularly for a couple of years now, I did keep fish when I was younger but just got into planted tanks and aquascaping recently. Anyway, I wanted to ask a request of you and also this may be a good topic for you to cover on your podcast. I know that you do critiques and analysis on aquascapes on your blog. But it may be nice to maybe review some amateur scapes to help people out. I come from an artistic background and when I saw these beautiful scapes online, when I was doing my research, I was amazed and instantly hooked. But it is very hard, as you know to get some good advice, especially online. Anyway, maybe this is something that you think could be interesting, I thought I’d throw it out there. You can maybe start with my scape from last year. I submitted it into the IAPLC for this year. Its since been taken down, I started a new one in the same tank this year.
Next up is an email from Dan. He writes:
I have kept what I have recently learned is called a low tech tank for over 20 years off and on never got into the whole high tech thing. I dabbled in info with the optimum aquarium decided that was too much work but I have always kept crypts and anubias mainly because my local lfs that’s all he carried I hate big box stores give me a mom and pop shop any day. Anyways, about 2 years ago I joined my local fish club GCAS that I knew a lot was I wrong but I have learned a lot from them as well as made some great friends and connections well this past weekend one of my GCAS brothers asked if I ever thought about doing co2 again with a family I didn’t want to spend hundreds on a system well he gave me a great deal
and another buddy told me about the lcd lights he used so for less than 200 for lights fully automated system with that being said I was referred to plantedtanks.net stumbled across a post that had info on your podcasts. Well, I downloaded all 12 and up to episode 10 couldn’t stop listening I even missed my reds game on the radio. I absolutely enjoy your casts. I would like to make a request unless its in episode 11 or 12 I would like to hear more on dosing and aquascaping dos and donts I know that could take hours.Again, thank you very much I have learned several thing from you podcasts as well as some new avenues to look into thank you for all you do to promote and keep the planted tank alive. I will never put plastic in the aquarium again!
Lastly, Pawel writes:
Hi,
I’m writing because I wanted to thank you for the work you’ve done. I really appreciate it – it makes time at work pass quicker and I can learn something about planted tanks. I wanted to thank you for the link to DFW Aguatic Plant Club, they have great article for noobies by Ben Belton.
I live in Poland. I had an aquarium since I can remember and since I can remember there were plants in it. Right now I have a 120 L (30 gal) communal tank, it is fairly low tech and low cost. There is a chinese canister filter, DIY CO2 system witch “yeast brewery” and for lightning thing up two T8 and one T5 fluorescent bulb (I’m attaching a photo of a tank). At the beginning I was concentrated on fishes but my interests shifted to plants on the way, to the point that recently I am building a planted paludarium. Well maybe I’ve said to much because for now I have only a DIY stand (photo included), but I certainly will build it sooner or later. I’m taking my time and considering a lot of options so the work is slow.
I have some questions for you:
1. I will be documenting this “making of” paludarium. It will be DIY and preferable low cost project. And here comes the question: Would you like to receive that documentation in order to post it on your site? Maybe someone could benefit from it. I’m not interested in creating a jungle of plants rather something more aesthetically pleasing, but I’m not so sure if it could be considered a scape or not, that will be up to you to tell.
- What do you think about LED lightning systems? (Or maybe you’ve already have that topic covered, I haven’t listened to the latest episode.)
- What do you think about having a riparium with fast growing plants on reversed overlapping photo-periods connected to the main tank. (In other words a planted sump tank). My thinking is as fallows: When the main tank produce CO2 the sump uses it and produce O2 and the other way around. Then you can have CO2 dosed 24/7 without worrying for the build up. Having fast growing plants in a tank witch is hidden in the closed means that they uses up the excess of fertilizers from the main tank and you don’t have to trim it ever so often because the sump can look like.. whatever it looks like… and what is more you can have less water changes.
- I remember that there were times that I really didn’t have much time for the tank and then I tended to just cut on feeding fishes (witch were a few) and fertilizing. Plants were growing slower but I did something like one 30% water change every two months and things were well. There were no algae (except few little green spots here and there mainly on the glass and a few grayish brushes mainly on the philter witch wasn’t a container philter, but who had a container philter back then). On the other hand you are speaking of 50% water changes every week. Could you compere those two?
All the best and once more I really appreciate your work.
Next week’s episode
Next week on the ScapeFu Podcast, I hope JJ and Juris will be back flying the plane with me! We’ll get an update from Juris on his trip to Interzoo. I know he had a good time, I saw of picture of him with Mike Senske and George Farmer on Facebook. We’ll focus on aquascaping next time, we’re going to talk about the use of color in your aquascape. As usual, we’ll also have Aquascaping News, From the Forums and Focus on You.
If you liked this episode, please leave us a written review in iTunes and mention us the next time you’re in the forums. We do no advertising so it’s the only way we have to spread the word about ScapeFu. It’s VERY much appreciated.
If you’d like to give us some feedback, you have choices:
- You can reach me, Art, via email at art@scapefu.com
- Twitter at @ScapeFu
- Facebook at Art Pennom
- If you want to be on the air, leave us feedback via our voicemail system by clicking on the tab on the right hand side of ScapeFu.com
- Our ScapeFu Feedback page
Lastly, please subscribe to the ScapeFu Podcast so that you won’t miss an episode: iTunes , Stitcher, Soundcloud
Speak soon!
Art
Some reference links that made this episode possible:
- americanaquariumproducts
- aquariumlife
- aquariumslife
- aquariumslife 2
- aquaticplantcentral
- barrreport
- barrreport 2
- barrreport 3
- barrreport 4
- barrreport 5
- fishchannel
- plantedtank
- plantedtank 2
- plantedtank 3
- plantedtank 4
- plantedtank 5
- practicalfishkeeping
- scribd
- seachem
- skepticalaquarist
- thekrib
- ukaps
- youtube
- youtube 2
Podcast: Play in new window | Download (46.2MB)
Subscribe: iTunes | Android | RSS


2 Comments on “ScapeFu015: Measuring CO2 in Your Aquarium”
Hey Art, your PH/KH table above has wrong labels for “too much co2” and “not enough co2”, they should be inverted 🙂
Thanks for your work here!
Fab.
Thanks, Fab. I’ll look into this and correct it.